A synthetic Escherichia coli communication system mediated by extracellular cyclic AMP
Author cheating statement (2023)
This publication has never been published in the peer-review system. It is published here, on this website, more than 10 years after the writing of the manuscript. The identity of the author (or the authors) is hidden for reasons unrelated to science. In this publication, one will find description of experiments, figures, data… I have to warn the reader that I mainly cheat for all figures, data proposed here… I mean that I have performed a lot of arbitrations that would, without any doubt, be considered by most scientists as falsification of data/figure/experiment and thus as cheating. However, I always did it in order to maximise the probability that the reader succeeds to reproduce the results and, thus, thinks internally: “what this Man has claimed is actually the truth”. I repeat that the anonymisation of the first author of this publication is unrelated to this confession of cheating.
One scientist helps me a lot with experiments with the mice. I have told her that she would be associated as author in this publication and I still want it. However I have to judge that she would probably not want to be associated in any way with the content of this website. Nevertheless, if she finds this publication and wants to be associated as author (I mean if I’m wrong), I would be very proud to add here name as second author.
Viafx24
Abstract
In Escherichia coli, intracellular cAMP is involved in carbon catabolite repression. It binds to the cAMP receptor protein (CRP) to control the transcriptional of more than 220 operons. Escherichia coli also actively exports cAMP into the growth medium where this molecule reaches non-negligible concentrations. However its role is not understood. In this study, we have engineered strains capable of communicating with each other by way of extracellular cAMP. We propose this new communication module to the synthetic biology community and we discuss its potential physiologic role in natural environment.
Introduction
3'-5'-cyclic adenosine monophosphate (cAMP) is a second messenger performing intracellular signaling in many organisms. Discovered in the 60s, the importance attributed to this compound has never stopped growing (Makman & Sutherland, 1965). The number of publications involving cAMP now exceeds 100 000. In humans, the intracellular cAMP-dependent pathway is involved in the regulation of many biological processes ranging from glycogen metabolism to synaptic transmission and the control of heart rate. The deregulation of concentrations of intracellular cAMP is observed in many diseases such as cholera, anthrax, cancers or neurological disorders.
In addition to its role as an intracellular second messenger, cAMP also acts as first messenger (Konijn et al., 1967). This function of cAMP was first discovered in the amoebae Dictyostelium discoideum, where extracellular cAMP mediates communication between cells by acting as a chemo-attractant. Periodic waves of extracellular cAMP allow these amoebae to convert from solitary predators into gregarious community members (Tomchik & Devreotes, 1981). In the 1970s, some reports mentioned that cAMP was actively exported in the blood of humans (and then urine) where it could attain significant concentrations (in the range 10-100 nM) (Broadus et al., 1970). Thirty years later, a wide body of experimental observations suggests that extracellular cAMP exerts endocrine and paracrine actions in several tissues (Bankir et al., 2002).
In Escherichia coli, cAMP is involved in carbon catabolite repression (Gorke & Stulke, 2008; Narang, 2009) and binds to the cAMP receptor protein (CRP). The corresponding complex is a transcriptional factor controlling the expression of more than 220 operons (Keseler et al., 2009). It has been known for a long time that E. coli actively exports cAMP into the growth medium (Goldenbaum & Hall, 1979; Makman & Sutherland, 1965). However, the role of this extracellular cAMP is not understood. In this study, we have engineered strains capable of communicating with each other by way of extracellular cAMP. Even though we have engineered the strains to clearly show communication via cAMP, the same mechanism could operate in a natural setting and provide a mean for communication between species that are phylogenetically only very distantly related.
Results
Communication on solid medium
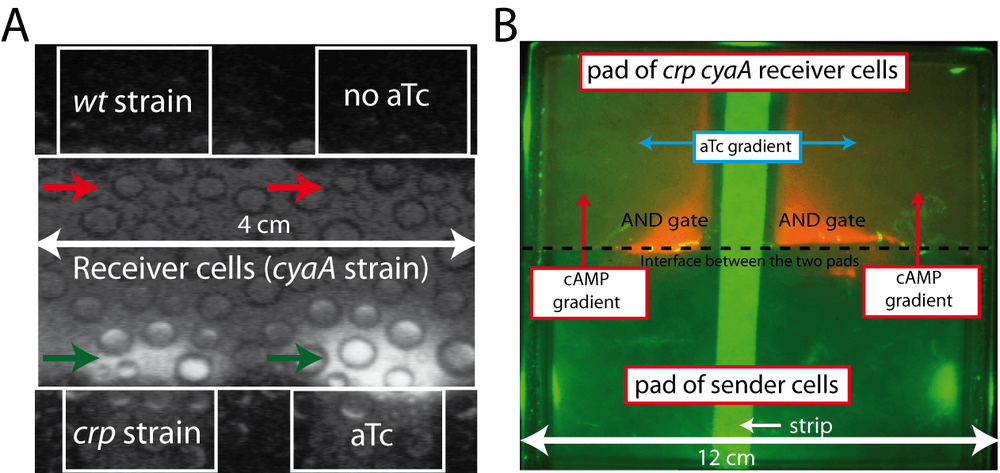
To build an inter-cellular communication system, we engineered a sender strain capable of producing more than the usual amount of cAMP and a receiver strain able to sense this exported cAMP and to report its presence by a convenient signal. The simplest sender strain is the crp mutant, known to produce high extracellular cAMP level compared to a wild-type strain (Potter et al., 1974). The receiver strain harbors a deletion of the cyaA gene (encoding the cAMP biosynthetic enzyme adenylate cyclase) in order to prevent any cAMP production. This cyaA mutant also contains a "bio-sensor" that converts the cAMP concentration into a luminescence signal. Placed on a plasmid, this biosensor is a transcriptional fusion, i.e, a promoter fused to a reporter gene. We chose the Crp-cAMP dependent sdhCDAB promoter (Nam et al., 2005) and fused it upstream of the luxCDABE operon coding for bacterial luciferase (Van Dyk et al., 2001).
To demonstrate inter-cellular communication, we used an interface assay. We embedded the sender cells into agarose gel containing minimal glucose medium on a Petri dish. The solid agarose gel containing the sender bacteria is brought into contact with a similar pad containing receiver cells. The Petri dish is placed in a heated, dark chamber and filmed for 40 hours using a sensitive CCD camera. An intense "halo" of luminescence appears in the area of contact between the "receiver cells" and the crp strain, called "sender cells" (Figure 1A; bottom left side). cAMP produced by the "sender area" has diffused into the "receiver area" and elicited the expected response: entering the receiver cells, binding to Crp and activating the sdh promoter, thereby inducing the expression of luciferase. By contrast, no "halo" appears at the interface between receiver cells and a wild-type strain (Figure 1A; top left side). This observation shows that a wild-type strain does not produce a sufficient amount of extracellular cAMP to allow the detection of communication.
Communication could be due to the exchange of any molecule, not necessarily cAMP. In order to demonstrate that cAMP is the signaling molecule, we remove the gene coding for adenylate cyclase (cyaA) in the sender strain. We put into the cyaA crp double mutant a plasmid that expresses cyaA from an anhydrotectracycline (aTc) inducible promoter. This strain produces cAMP only when the inducer aTc is present in the medium. As expected, a halo of luminescence appears only at the interface of the pad containing aTc (Figure 1A; right bottom side versus right top side). This experiment demonstrates that the expression of adenylate cyclase in the sender strain is necessary for inducing transcription of luciferase in the receiver strain.
A second way to demonstrate that cAMP acts as an intercellular signaling molecule is to show that the presence of the crp gene in the receiver strain is needed for the appearance of the luminescence halo. We transformed a cyaA crp double mutant with the plasmid PR100, carrying a construct that over-expresses cyaA. This latter is under the control of the strong lambda pL promoter and the gene carries the canonical AUG initiation codon, instead of the less efficient TTG found in the chromosomal cyaA gene. With this expression system, adenylate cyclase constitutes approximately 30% of the cellular proteins. As a receiver strain we also use the cyaA crp double mutant containing two plasmids. The first one carries the luminescence biosensor and the second plasmid allows to tune the expression of Crp by the aTc inducible system. We juxtaposed two agar pads containing these two strains and we added, overlaid in the middle of the Petri dish, and perpendicular to the generated interface, a thin strip of paper soaked with a solution of aTc. Figure 1B shows that in the area where the aTc gradient (expression of Crp) and the cAMP gradient generated by the sender cells overlap, we observe a bright luminescence of the reporter plasmid. Activation of the reporter gene corresponds to the AND logic gate behavior of the Crp-cAMP complex. Communication passes through the first messenger cAMP produced by sender cells and requires the Crp protein as a second component in the receiver cells.
Tuning the communication in liquid medium
We have shown that communication via cAMP functions on solid medium. Is the amount of excreted cAMP sufficient to produce communication in liquid medium? In order to obtain quantitative information, we decided to duplicate the reporter system. In addition to the luciferase reporter, we added to the receiver strain an equivalent gfp reporter, also driven by the sdh promoter (Zaslaver et al., 2006), but carried on a plasmid with a different origin of replication (pSC101). We measure the sdh promoter activity independently with each reporter system in the same cell (Figure 2A). This double reporter system eliminates all potential artifacts due to the reporter system or the plasmid.
We first tuned the strength of communication by controlling the expression of adenylate cyclase in the sender (Figure 2A; see also the tridimensional simulation in supplementary video 1). We grow sender cells (allowing the control of cyaA expression by aTc) and receiver cells in a minimal glucose medium for 24 hours. The culture grows in a microplate and we continuously monitor luminescence, fluorescence and optical density. At specific points of the kinetics, we collected samples to measure extracellular cAMP by an immunoassay kit. Figure 2B shows that the extracellular cAMP concentration is proportional to the aTc level controlling adenylate cyclase expression in the sender cells. The increase of extracellular cAMP over time is gradual and reaches 80 μM after about 20h of growth.
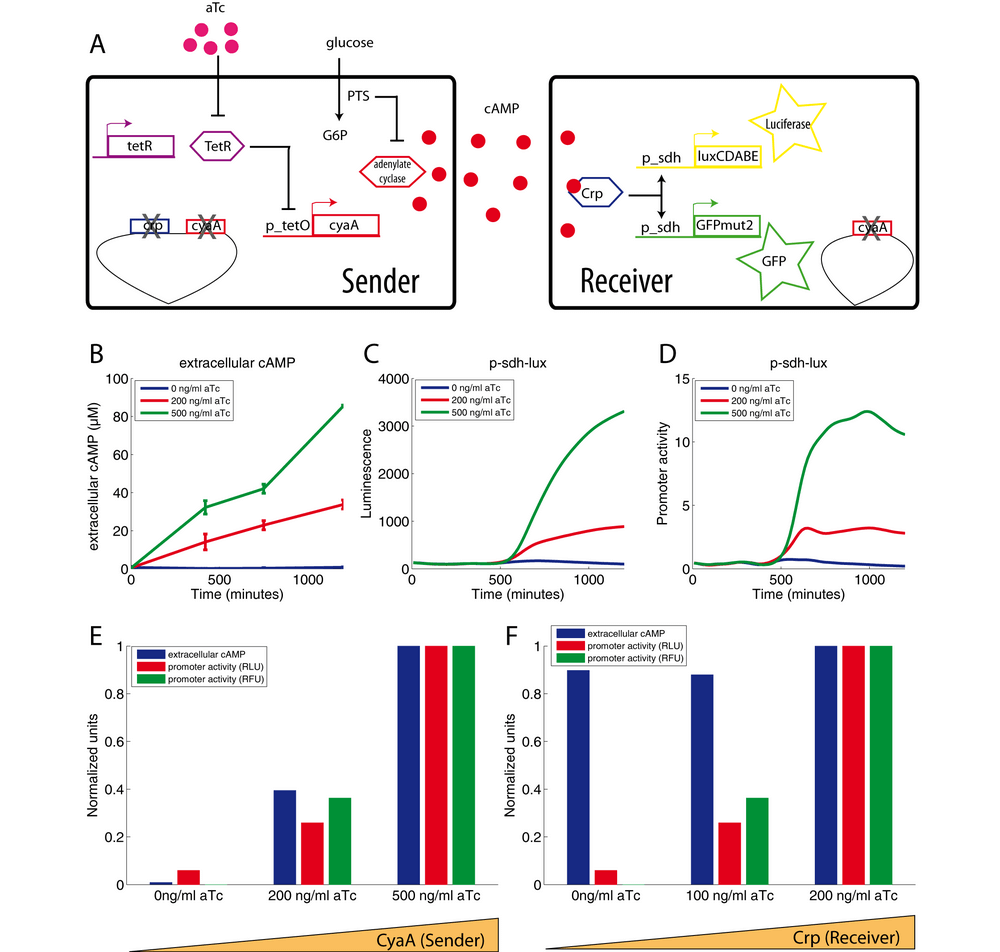
Figures 2C and 2D show the corresponding induction of the sdh promoter after glucose exhaustion (at about 550 min). Both reporter systems, fluorescence and luminescence, show the same signal. This experiment shows that we can control the transcription of luciferase and GFP in receiver cells by tuning the adenylate cyclase level in the sender cells. Last, contrary to the gradual increase of extracellular cAMP, the strong increase of fluorescence and luminescence occurs only after glucose exhaustion (growth arrest indicated by an arrow on the figure 2C). This result does not fit well with the classic model of carbon catabolite repression. Indeed, adenylate cyclase in sender cells should produce cAMP only after glucose exhaustion because its activator (the phosphorylated form of EIIAglc.) is supposed to be present only in absence of glucose (Park et al., 2006). Yet, the raise of extracellular cAMP is not linked to the glucose exhaustion and cAMP is already present at a high concentration in the medium well before sdh activation at 600 minutes (Figure 2B versus Figures 2C and 2D). This result means that the repression of sdh promoter activity by glucose is not due to the inactivation of adenylate cyclase in sender cells but rather due to an unknown mechanism that occurs in receiver cells.
Another way to tune the strength of communication consists in controlling the Crp concentration in the receiver cells (figure 2F). In this experiment, we used sender cells carrying the PR100 plasmid, which produces large amounts of cAMP. This microbial factory generates an extracellular cAMP concentration close to 150μM (data not shown). The fluorescence and luminescence levels follow the Crp concentration in the receiver cells (range of aTc) (Figure 2F) but only after glucose exhaustion. Once again, sdh promoter activity is repressed by a strong glucose repression. This latter is neither related to variation of extracellular cAMP level nor related to variation of Crp level. Another explanation must be sought.
Recovery of motility through communication
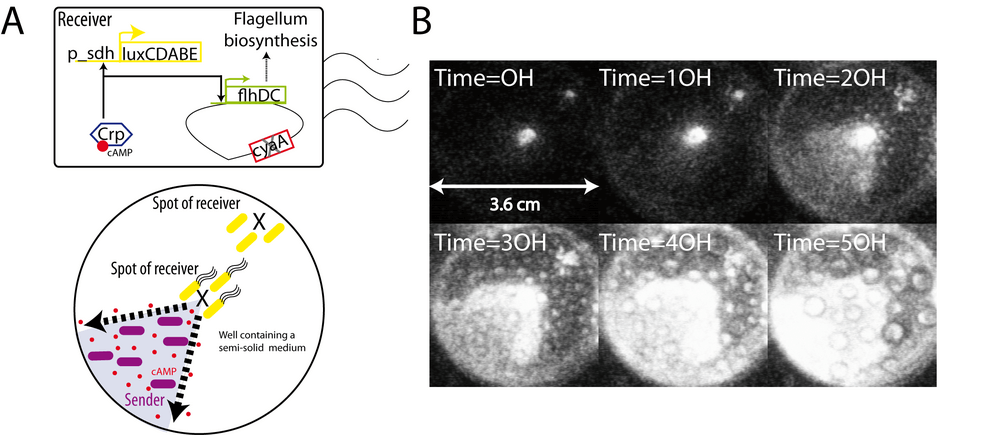
How could communication be used to elicit a physiological response? The cyaA mutant has many characteristic phenotypes. A cyaA mutant cannot utilize carbon sources such as maltose, lactose or acetate and it cannot produce flagella, resulting in a non-motile bacterium. This lack of motility is due to the absence of FlhDC, the principal regulator of bacterial flagellum biogenesis. The transcription of the flhDC operon is positively regulated by the cAMP-CRP complex (Soutourina et al., 1999). If communication works, cAMP produced and exported by sender cells should complement the non-motile phenotype of cyaA receiver cells. We designed an assay based on the faculty of Escherichia coli to swim though a semi-solid medium (low concentration of agarose). We filled 3/4 of a well (6-wells microplate) with this semi-solid gel. The bottom left quarter also contains sender cells. Two drops of receiver cells where spotted onto this agarose gel: one at the center of the well close to sender cells, a second spot at the top left, far from sender cells (Figure 3A). We filmed the microplate for 50 hours and observed that the "center" spot spreads to the bottom left quarter containing sender cells as shown by luminescence (Figure 3B). By contrast, the "top left" spot does not move. We conclude that cAMP produced by sender cells complements the cyaA strain and allows the recovery of motility probably by allowing flhDC expression followed by flagella biosynthesis. Too far from cAMP, cells of the second spot do not recover motility. It has not escaped our attention that this system can be viewed as a synthetic guidance system.
Discussion
A synthetic system
In this study, we have developed an easily tunable synthetic system allowing inter-cellular communication of Escherichia coli cells by the way of extracellular cAMP. We propose this system to the synthetic biology community and discuss these advantages and disadvantages. Contrary to other intercellular communication systems such as LuxI/LuxR from Vibrio fischeri or LasI/LasR from Pseudomonas aeruginosa (Balagadde et al., 2008), the main default of our system is its absence of orthogonality. Indeed, it is not independent from the regulatory network of E. coli. On the contrary, the cAMP-CRP complex is considered as a "hub". The lack of orthogonality places our system at the edge between synthetic and system biology. Because our system is not uncoupled from natural regulatory network, it can be used as a tool to understand, disturb and control it. The huge knowledge on the cAMP-CRP complex can be used and embezzle to develop new synthetic functions: The high number of promoters controlled by Crp-cAMP offers a wide choice of different affinity binding site (Keseler et al., 2009). The "hub" Crp-cAMP is already connected to a lot of different cellular modules (motility, chemotaxis, virulence, biofilm, stress resistance, carbon utilization…) and composes a wide number of known network motifs such as coherent and incoherent feed forward loops (Alon, 2006). The connection of the Crp-cAMP "hub" to metabolism, via the mechanism of carbon catabolite repression, is a case study for system biology and different already existing models could be re-used (Bettenbrock et al., 2006; Hardiman et al., 2007; Kotte et al., 2010; Nishio et al., 2008). Last, cAMP is strongly ubiquitous: it's a messenger shared by most of the living species. Extracellular cAMP is thus a good candidate to perform synthetic inter-kingdom communication systems.
A microbial factory and a biosensor as new tools
In this study, we engineered a strain able to produce up to 150μM extracellular cAMP i.e well above the concentration reached by a wild-type strain (100nM-10μM) (Matin & Matin, 1982). It's still far from the concentration (30mM) produced by Microbacterium sp. no. 205 (Ishiyama, 1990). It is therefore unlikely that our strain is competitive on an industrial scale. However the current yield can probably be optimized by adapting the growth conditions or by strain improvement, for instance, by removing the phosphodiesterases (cpda) which has a reported Km of 500μM (Imamura et al., 1996) or by modifying the phosphate transfer system to improve adenylate cyclase activation (Gosset, 2005).
In this study, we also described a "biosensor", a cyaA strain that convert exogenous cAMP concentration into luminescence and fluorescence signals. The lower limit of detectable concentrations is closed to 10μM. The active export of cAMP is probably the main reason explaining why the biosensor fails to easily report extracellular cAMP concentration yet equivalent to physiologic intracellular concentration in a wild-type strain. Removing this mechanism of export may increase the sensitivity of our biosensor to reach the Crp km. Last, the unknown phenomenon of glucose repression observed in the figure 2 but unrelated to cAMP concentration is a parameter that influences the output signals generated by our reporters. Understanding and removing this phenomenon may also increase the performance of the biosensor.
[2023: Several months after the writing of this manuscript and PhD defence, I have built a cya- cpda- double mutant that appears to be a much sensitive “receiver strain” to extracellular cAMP. From memory, I wan a factor 10 in sensitivity. The video of communication “at the single cell level” proposed at the end of this manuscript has used a cya- cpda- receiver strain.
A potential physiologic system
In this study, we do not demonstrate that communication by cAMP is a physiologic system in Escherichia coli. Indeed wild-type cells produce a maximum extracellular cAMP level (5-10μM) which roughly corresponds to the minimum threshold of detection of our biosensor. Is that mean that the reachable extracellular cAMP concentration must not change drastically the gene expression of the population? But then why is cAMP exported in the medium? It has already be demonstrated that the cost of the cAMP export in term of ATP molecules is too high to only serve as a mechanism used to regulate intracellular cAMP level (Matin & Matin, 1982). Could we imagine that a tiny fraction of the population begins to sense the extracellular cAMP and expresses Crp-cAMP dependant operon as a bet hedging strategy? is E. coli uses its secreted extracellular cAMP to communicate with other species or less "anthropocentrically" to change their phenotypes? One track to search for physiologic communication process may be to look towards the natural environment of E. coli: the intestinal ecosystem. By way of conclusion, we invite the reader to watch the Video 2 (and the explanations in supplementary information) where we made an attempt in this direction.
Materials and Methods
Strains and plasmids
We used the E. coli K-12 strain BW25113 as "wild-type strain". This strain has a well-defined pedigree and has not been subjected to mutagens (Datsenko & Wanner, 2000). We used the cyaA- strain and the crp- strain of the Keio collection (Baba et al., 2006). We removed the kanamycin resistance cassette of the cyaA- strain using flp recombinase in order to allow utilization of the p-sdh-gfp plasmid which also harbors a kanamycin resistance cassette (Zaslaver et al., 2006). The cyaA- crp- double mutant was constructed by P1 transduction of the spectinomycin resistance cassette of another crp- mutant (Soutourina et al., 1999).
Precultures were always performed in LB medium with the appropriate antibiotics (ampicillin 100 μg/ml; kanamycin 50 μg/ml; chloramphenicol 30 μg/ml; spectinomycin 10 μg/ml) at 37°C under shaking with the exception of the strain harboring the PR100 plasmid (with the thermosensitive lambda promoter) which has grown at 30°C to prevent too strong expression of cyaA resulting in insoluble adenylate cyclase (Reddy et al., 1989).
The gltA-sdhC intergenic region was amplified by PCR using primers 5'-TATCCTCGAGTTAAGGTCTCCTTAGCGCCTT-3' (xho1 site underlined) and 5'- TTCTGAATTCGAATAACGCCCACATGCTGTTC-3' (EcoRI in bold) using BW25113 strain as template and inserted into luxCDABE plasmid backbone without promoter to make p-sdh-lux.
The crp gene was amplified by PCR using primers 5'-ATAAGGTACCATGGTGCTTGGCAAACCGC-3'(kpnI site underlined) and 5'-TCCGGGATCCTTAACGAGTGCCGTAAAC-3'(bamH1 site underlined) using BW25113 strain as template. The PCR product, digested with kpn1 and bamH1, was inserted into the previously described p-tet-lux plasmid to make p-tet-crp.
The cyaA gene was amplified by PCR using primers The 5'-GCGAGGTACCTTGTACCTCTATATTGAGACT-3' (kpnI site underlined) and 5'-ACAGTCGCTAGCTTAACTAGCATCAGCATAATTTTCACTGTAGTTTTCGTCGTTTGCTGCCGAAAAATATTGCTGTAATAGC-3' (nheI site underlined) using BW 25113 strain as template. The PCR product, digested with kpn1 and nheI, was inserted into the previously described p-tet-lux plasmid to make p-tet-cyaA. Note that the oligo introduces a degradation tag das+4 in 3' (AANDENYSENYADAS). This tag doesn't play any role in this study but it may significantly reduce the half-life of the corresponding adenylate cyclase (McGinness et al., 2006). The PR100 plasmid was a gift from Pr. Reddy.
Solid media assay
We used a minimal medium with 0.3% (w/v) glucose and 0.8% (w/v) low melt agarose. After a washing step to remove antibiotics, we added, in the unsolidified medium and just before polymerization, the receiver cells (dilution factor 1/10) or the sender cells (dilution factor 1/4) coming from LB pre-cultures. Interfaces were created step by step by pouring the unsolidified medium mix in the Petri dish (then sometimes, cutting to adjust final shape) and waiting for solidification. Then the next unsolidified medium mix was added so as to touch the previous pad to create the interface. After polymerization of the entire pad, the Petri dish was placed in a heated chamber and filmed during the appropriate time (days) by a sensitive CCD camera (photonic science). The thin strip of paper (whatman) in Figure 1 is saturated with 100 μl of a solution of anhydrotetracycline (1mg/ml).
Liquid media assay
Dynamic analyses were performed by growing cells during 24 hours in a 96-well microplate. The microplate reader (fusion alpha FP-HT) allows growth with shaking and temperature control (37°C). It measure cell density (OD at 600nm), luminescence or fluorescence (480nm/520nm) every 15 minutes. Each well contains 180 μl of minimal medium (M9) with 0.3% glucose (w/v) and with a range of anhydrotetracycline (0, 100, 200, 500 ng/ml). We added 10 μl of appropriate receiver cells and 40 μl of appropriate sender cells after a washing step to remove the antibiotics of the preculture (LB). Contrary to luminescence data which don't produce background, we subtracted a background from fluorescence data: this background is the fluorescence generated by the control (0 ng/ml aTc). Sample for extracellular cAMP measurement were collected at 0, 420, 750 and 1200 minutes. We used the chemiluminescent cAMP HTS immunoassay (Millipore). All measures were performed in triplicate except one outlier removed (at time 1200, 500 ng/ml, Figure 2).
Semi-solid media assay
The minimal medium (M9) contains 0.3% galactose and 0.3% low melt agarose. We added 4ml in a well of a 6 wells-micro plates. Once "semi-solidified", we were still able to sculpt the agar and we removed, by using a pipetman, one quarter of the semi-solid medium (0.8 ml) that we replaced by 0.8ml of the same medium but containing the sender cells (crp-cyaA- double mutant with the PR100 plasmid) (a pullet resuspended coming from the centrifugation of 2ml of preculture). Then we added, on the top of the agar, two spots (center and top left) of 1μl of receiver cells (concentrated 200X).
Image processing
All movies and images were processed using Matlab software. No specific image enhancement has been performed.
Supplementary information
Calibration of the p-sdh-lux biosensor
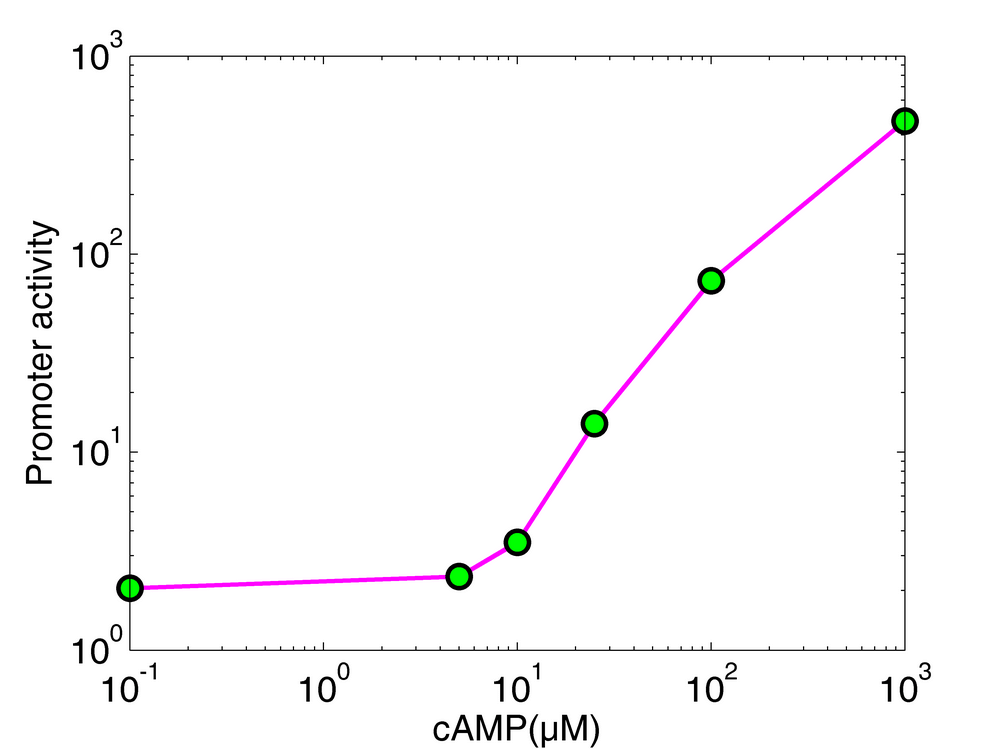
We measured the luciferase activity of the p-sdh-lux plasmid during growth in a microplate reader (37°C with shaking). Each well contained 180 μl of minimal medium (M9) supplemented with glucose (0.3%) and the indicated concentration (0, 5, 10, 25, 100, 1000 μM) of cAMP. We added 20 μl of a LB pre-culture (cyaA- strain transformed with the p-sdh-lux luminescence biosensor) to each well at time zero. The promoter activity, computed from luminescence data, was calculated from the expression profile after the glucose exhaustion. The minimal detectable cAMP concentration of this biosensor, corresponding to a measurable difference in promoter activity, is around 5-10μM.
Stochastic simulation of the communication
We have implemented a stochastic, three-dimensional model to simulate the communication process. The code has been written in Matlab and is available upon request. Although this model reproduces the observed patterns, it should rather be considered as didactic. We do not derive any parameters from this simulation.
The screen of the video is divided in two parts. On the left, the video schematizes the different steps of the communication process. On the right, camera rotations and zooms highlight the tridimensional space and the events that we describe below. Each screen corresponds to a distinct simulation. Consequently, due to stochasticity in gene expression, the two simulations are not completely synchronous. At the beginning of the video, one can see three yellow ellipsoids that represent three cyaA- receiver cells. The three pink ellipsoids represent the crp- senders. All cells harbor a red, super-coiled plasmid. The sender cells produce adenylate cyclases (dark blue cube) that move randomly inside the cell and synthesize cAMP (light blue cube). Contrary to the proteins, cAMP progressively diffuses into the external medium, enters the receiver cells and binds to Crp proteins to form a cAMP-CRP complex. The molecular interactions, too computationally expensive, are not modeled. When cAMP molecules exceed a certain threshold in the receiver cell, there is a certain probability that CRP changes its conformation (cube to sphere), increasing its affinity for binding site on the plasmid. Once bound to the DNA (watch the event on the right screen), the synthesis rate of luciferase increases, allowing transcription and traduction of luxCDABE and subsequent appearance of luciferase molecules (green cube) in the receiver cells. This event signals the success of the communication procedure. The receiver cells become green at different time due to stochasticity in gene expression. Obviously, those temporal differences can not be observed in our experimental data (presented in figure 2), obtained at the population level.
Investigation of the mouse gastro-intestinal ecosystem
We wanted to test whether communication by way of extracellular cAMP could occur between E. coli and other species in their natural environment: the gastro-intestinal tract. Unfortunately, we could not answer this particular question. The experiment shown here nevertheless demonstrates that external cAMP can induce gene expression in E. coli within the gastro-intestinal tract. It has previously been shown that the light produced by an E. coli population that has invaded the intestine of a mouse can be detected "transcutaneously" by a sensitive CCD camera. In our experiment, we administered orogastrically 200μl of a solution containing approximately 25 billion cyaA- bacteria (containing the luminescence biosensor) to two mice. After 30 minutes, we administered orogastrically 250μl of LB containing 100mM cAMP to one mouse and 250 μl of LB to the second mouse (control). We then anesthetized the mice and placed them in a dark chamber under a sensitive camera to acquire luminescence images. Movie 4 shows that the expression of a gene (luxCDABE) is switched on in the gastro-intestinal tract of the mouse that has received cAMP. The mouse on the right, that drank the LB + cAMP solution, emits a strong luminescence detected transcutaneously and thus in a non invasive way. The mouse on the left, that drank LB as control, produces no light. We have reproduced this experiment twice.
Materials and methods
Briefly, two Swiss CD-1 mice (Janvier, Le Genest-Saint-Isle, France) were given drinking water containing 500 μg/ml of kanamycin and ampicillin for 16h to kill most commensal bacteria. The cyaA strain containing luminescence and fluorescence biosensors was grown in 100 ml of LB medium (with 100 μg/ml ampicillin and 50 μg/ml kanamcyin) overnight at 37°C under shaking. We resuspended the cells in 2 ml of LB and we administered orogastrically 200 μl of this solution with a feeding needle. 30 minutes later, we administered 250 μl of LB 100 mM cAMP to one mouse and 250 μl of LB to the second mouse (control). The mice were then anesthetized with a mixture of ketamine hydrochloride (Imalgen 500, Merial, 25 mg/kg body weight) and xylasine (Rompun, Bayer Healthcare, 12.5 mg/kg body weight), injected intra-peritoneally . The two mice were placed in a heated dark chamber and filmed for several hours by a sensitive CCD camera. Luminescence images were acquired every 30 seconds. We had to anesthetize the mice three times during the kinetics. This explains why the luminescence spot moves in the film just before the next anesthesia: a mouse begins to awake. The images of mice do not detect the awakening because there are static images taken at the beginning of each anesthesia. These images were subsequently overlaid onto the luminescence images (red false color). The movie was created using Matlab. No specific image enhancement has been performed.
Ethical statement
Animals were housed according with the French Ethical Committee (Decree 87-848) and European Community Directive 86/609/EEC. Experiments were carried out under the supervision of CL (agreement nu 38 08 08) in the animal care facilities approved by the Direction des Services Vétérinaires de l'Isère (Nu A 38 516 10006).
Communication at the single cell level
[2023: this experiment was not present in this publication/thesis. I have performed it several months after thesis defence (october 2012). I have decided to add it here because, at this time, I was a bit “proud” about this little success! 😀]
Since I’m writing this paragraph in 2023, I don’t precisely remember the experimental protocol. However, things were probably simple. The "red" strain was probably the sender strain (with to plasmids, one for overexpression of cyaA and one for overexpression of the red fluorescent protein). The “green” strain was probably the receiver strain with deletion of cyaA and cpdA and with a reporter plasmid that express the green fluorescent protein only if cAMP is present.
The left side of the video shows development of microcolonies mainly of senders (red; RFP). Once the glucose is exhausted, the microcolonies stop their exponential growth and the receiver strains, less numerous, begin to become green, expressing the GFP. This is possible because there is sufficient cAMP exported in the medium by the sender strain. This GFP expression demonstrates the “communication”. Indeed, in the control video concatenated on the right side, there is only the receiver strain (the same strain that the one used on the left side) and the microcolonies never become green because it lacks cAMP.
References
- Alon, Uri. 2006. An Introduction to Systems Biology: Design Principles of Biological Circuits (Chapman & Hall/CRC Mathematical & Computational Biology). 1 edn. Chapman and Hall/CRC.
- Baba, T., Ara, T., Hasegawa, M., Takai, Y., Okumura, Y., Baba, M., Datsenko, K. A., Tomita, M., Wanner, B. L., & Mori, H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol, 2, 2006 0008. Gm62662/gm/nigms Journal Article Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t England.
- Balagadde, F. K., Song, H., Ozaki, J., Collins, C. H., Barnet, M., Arnold, F. H., Quake, S. R., & You, L. 2008. A synthetic Escherichia coli predator-prey ecosystem. Mol Syst Biol, 4, 187.
- Bankir, L., Ahloulay, M., Devreotes, P. N., & Parent, C. A. 2002. Extracellular cAMP inhibits proximal reabsorption: are plasma membrane cAMP receptors involved? Am J Physiol Renal Physiol, 282(3), F376–92.
- Bettenbrock, K., Fischer, S., Kremling, A., Jahreis, K., Sauter, T., & Gilles, E. D. 2006. A quantitative approach to catabolite repression in Escherichia coli. J Biol Chem, 281(5), 2578–84.
- Broadus, A. E., Kaminsky, N. I., Northcutt, R. C., Hardman, J. G., Sutherland, E. W., & Liddle, G. W. 1970. Effects of glucagon on adenosine 3’,5’-monophosphate and guanosine 3’,5’-monophosphate in human plasma and urine. J Clin Invest, 49(12), 2237–45.
- Datsenko, K. A., & Wanner, B. L. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A, 97(12), 6640–5. Journal Article Research Support, U.S. Gov’t, Non-P.H.S. United states. 14
- Goldenbaum, P. E., & Hall, G. A. 1979. Transport of cyclic adenosine 3’,5’-monophosphate across Escherichia coli vesicle membranes. J Bacteriol, 140(2), 459–67.
- Gorke, B., & Stulke, J. 2008. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat Rev Microbiol, 6(8), 613–24.
- Gosset, G. 2005. Improvement of Escherichia coli production strains by modification of the phosphoenolpyruvate: sugar phosphotransferase system. Microb Cell Fact, 4(1), 14. Journal article.
- Hardiman, T., Lemuth, K., Keller, M. A., Reuss, M., & Siemann-Herzberg, M. 2007. Topology of the global regulatory network of carbon limitation in Escherichia coli. J Biotechnol.
- Imamura, R., Yamanaka, K., Ogura, T., Hiraga, S., Fujita, N., Ishihama, A., & Niki, H. 1996. Identification of the cpdA gene encoding cyclic 3’,5’-adenosine monophosphate phosphodiesterase in Escherichia coli. J Biol Chem, 271(41), 25423–9.
- Ishiyama, J. 1990. Isolation of mutants with improved production of cAMP from Microbacterium sp. no. 205 (ATCC 21376). Appl Microbiol Biotechnol, 34(3), 359–63.
- Keseler, I. M., Bonavides-Martinez, C., Collado-Vides, J., Gama-Castro, S., Gunsalus, R. P., Johnson, D. A., Krummenacker, M., Nolan, L. M., Paley, S., Paulsen, I. T., Peralta-Gil, M., Santos-Zavaleta, A., Shearer, A. G., & Karp, P. D. 2009. EcoCyc: a comprehensive view of Escherichia coli biology. Nucleic Acids Res, 37(Database issue), D464–70.
- Konijn, T. M., Van De Meene, J. G., Bonner, J. T., & Barkley, D. S. 1967. The acrasin activity of adenosine-3’,5’-cyclic phosphate. Proc Natl Acad Sci U S A, 58(3), 1152–4.
- Kotte, O., Zaugg, J. B., & Heinemann, M. 2010. Bacterial adaptation through distributed sensing of metabolic fluxes. Mol Syst Biol, 6, 355.
- Makman, R. S., & Sutherland, E. W. 1965. Adenosine 3’,5’-Phosphate in Escherichia Coli. J Biol Chem, 240, 1309–14.
- Matin, A., & Matin, M. K. 1982. Cellular levels, excretion, and synthesis rates of cyclic AMP in Escherichia coli grown in continuous culture. J Bacteriol, 149(3), 801–7. 15
- McGinness, K. E., Baker, T. A., & Sauer, R. T. 2006. Engineering controllable protein degradation. Mol Cell, 22(5), 701–7. AI-16892/AI/United States NIAID Journal Article Research Support, N.I.H., Extramural United States.
- Nam, T. W., Park, Y. H., Jeong, H. J., Ryu, S., & Seok, Y. J. 2005. Glucose repression of the Escherichia coli sdhCDAB operon, revisited: regulation by the CRP*cAMP complex. Nucleic Acids Res, 33(21), 6712–22.
- Narang, A. 2009. Quantitative effect and regulatory function of cyclic adenosine 5’-phosphate in Escherichia coli. J Biosci, 34(3), 445–63.
- Nishio, Y., Usuda, Y., Matsui, K., & Kurata, H. 2008. Computer-aided rational design of the phosphotransferase system for enhanced glucose uptake in Escherichia coli. Mol Syst Biol, 4, 160.
- Park, Y. H., Lee, B. R., Seok, Y. J., & Peterkofsky, A. 2006. In vitro reconstitution of catabolite repression in Escherichia coli. J Biol Chem, 281(10), 6448–54.
- Potter, K., Chaloner-Larsson, G., & Yamazaki, H. 1974. Abnormally high rate of cyclic AMP excretion from an Escherichia coli mutant deficient in cyclic AMP receptor protein. Biochem Biophys Res Commun, 57(2), 379–85.
- Reddy, P., Peterkofsky, A., & McKenney, K. 1989. Hyperexpression and purification of Escherichia coli adenylate cyclase using a vector designed for expression of lethal gene products. Nucleic Acids Res, 17(24), 10473–88. Journal Article England.
- Soutourina, O., Kolb, A., Krin, E., Laurent-Winter, C., Rimsky, S., Danchin, A., & Bertin, P. 1999. Multiple control of flagellum biosynthesis in Escherichia coli: role of H-NS protein and the cyclic AMP-catabolite activator protein complex in transcription of the flhDC master operon. J Bacteriol, 181(24), 7500–8.
- Tomchik, K. J., & Devreotes, P. N. 1981. Adenosine 3’,5’-monophosphate waves in Dictyostelium discoideum: a demonstration by isotope dilution–fluorography. Science, 212(4493), 443–6.
- Van Dyk, T. K., Wei, Y., Hanafey, M. K., Dolan, M., Reeve, M. J., Rafalski, J. A., Rothman-Denes, L. B., & LaRossa, R. A. 2001. A genomic approach to gene fusion technology. Proc Natl Acad Sci U S A, 98(5), 2555–60. 16
- Zaslaver, A., Bren, A., Ronen, M., Itzkovitz, S., Kikoin, I., Shavit, S., Liebermeister, W., Surette, M. G., & Alon, U. 2006. A comprehensive library of fluorescent transcriptional reporters for Escherichia coli. Nat Methods, 3(8), 623–8.